Silicone vs. Saline Implants Los Angeles
 After 14 years of study, in late 2006 the Food and Drug Administration (FDA) declared silicone gel filled breast implants to be safe and effective. Plastic surgeons around the nation greeted the news with almost unanimous enthusiasm, welcoming the opportunity to offer their patients the choice between silicone and saline. In 2012, the FDA approved the fifth-generation Sientra® Silimed-brand implants – the original “gummy bears.” These highly anticipated form-stable implants represent the latest improvement in the continual evolution of silicone implants.
After 14 years of study, in late 2006 the Food and Drug Administration (FDA) declared silicone gel filled breast implants to be safe and effective. Plastic surgeons around the nation greeted the news with almost unanimous enthusiasm, welcoming the opportunity to offer their patients the choice between silicone and saline. In 2012, the FDA approved the fifth-generation Sientra® Silimed-brand implants – the original “gummy bears.” These highly anticipated form-stable implants represent the latest improvement in the continual evolution of silicone implants.
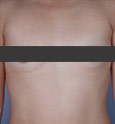
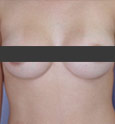
If you’re planning a breast augmentation procedure in Beverly Hills or Los Angeles, breast implants play an important role in your final results. How do you decide which implant to choose? Here you can begin to learn more about your implant options.
Silicone Implant Background
Breast implants with silicone filling were first manufactured in the early 1960s. Silicone’s popularity stems from two key advantages: it’s a material that is highly compatible with the body – finding uses in a variety of implantable medical devices such as artificial joints – and it creates breasts that have a very natural look and feel.
Silicone breast implants have been the subject of continued improvement and study over the years. The filling and shells have been modified again and again to upgrade both the safety and feel of the product. Nevertheless, in 1992 the FDA restricted use of silicone implants to just a small number of patients until further studies could be performed. The FDA declared silicone gel implants to be safe and effective and re-approved a new generation of silicone gel implants in November 2006. These are referred to as “fourth-generation” silicone implants.
Dr. Stevens and associates have always believed in the safety of silicone implants. Noting that there was no direct evidence the implants caused problems and that the devices were never banned in other countries, the surgeons offered them to their eligible patients when possible. In addition, Dr. Stevens was chosen to participate in FDA-sanctioned patient trials of new, high-strength cohesive silicone gel implants also known as “gummy bear” implants.
Silicone Implants Today
Fourth Generation Gel Implants
Worldwide, silicone gel has long been the preferred choice of women and physicians for the soft, natural look and feel that it imparts to enhanced breasts. Unlike saline, the cohesive silicone filling holds together if the shell should rupture, causing what the FDA terms a “silent rupture.” Though the material is not likely to migrate out of the breast pocket, the FDA advocates women have periodic MRI tests to ensure their implants remain intact.
Fourth generation silicone gel implants are available from 2 manufacturers: Mentor and Allergan. The products come in a variety of shapes, sizes and profiles, giving you and your surgeon the choices you need to create the perfect breast for you.
Fifth Generation Gel Implants
The FDA approved Sientra implants are filled with a high-strength, form-stable silicone gel that is the most cohesive ever available. The manufacturer reports that these fifth-generation implants have a 3 year rupture rate of 2% to 2.5%. Since the gummy bears don’t wrinkle or fold, and since wrinkles and folds contribute to shell failure, these implants will likely prove to have a longevity advantage.
A study published by Dr. Stevens found that these implants have a complication profile comparable to fourth generation silicone implants, with a lower rate of capsular contracture. Future studies will likely show that gummy bear implant ruptures, when they do occur, don’t lead to silicone migration. In demonstrations, these implants are cut in half, then squeezed, and the gel retains its shape much like a gummy bear would.
Dr. Stevens is one of only a handful of plastic surgeons in the country involved in the studies of fifth generation silicone gel devices popularly known as “gummy bear implants.” He has been heavily involved in the Sientra studies, and continues to participate in the clinical trials of the Mentor CPG’s, expected to receive FDA approval in 2013. As a participant in this study, Dr. Stevens has some options not available through other doctors. For the right candidates, these advanced silicone gel implants can offer important durability advantages while still looking and feeling like natural breast tissue. In addition, these implants can be chosen by women between the ages of 18 to 22 who are having their first augmentation, a group not currently eligible for the widely-available silicone implants. Also, through this study the surgeons are able to offer larger silicone implants than most other plastic surgeons, with implants up to 1,000 cc’s creating up to a DDD breast size.
Saline Implants
Saline implants were first manufactured in France in 1964 and appeared on the U.S. market in the 1970s. They gained FDA approval in 2000 and became the implant of choice when the silicone versions were restricted.
A key advantage of saline breast implants is the filling itself. Very similar to natural body fluids, saline is easily and harmlessly absorbed by the body should an implant rupture. And since the implant deflates, it’s easy to tell when a rupture has occurred and replacement is needed.
Saline implants are filled after placement into the breast pocket. This generally provides the patient with a slightly smaller incision and scar compared with pre-filled implants. In addition, because the implants can be filled with different amounts of saline once placed, our surgeons are able to precisely tailor the size of each implant in an effort to avoid breast asymmetry.
Disadvantages of saline implants are mainly cosmetic. Women are far more likely to report being unhappy with the way saline implants look, as they occasionally result in rippling and wrinkling. Some women believe this implant type does not feel like natural breast tissue, and occasionally patients complain of being able to hear “sloshing” of the solution inside the implant. A cosmetic as well as functional concern is increased saline implant deflation rates over time. Deflation requires surgical correction, though if deflation occurs, saline is harmlessly absorbed by the body and poses no health risk.
Saline implants also can be more difficult to use when a patient has limited tissue. Those who have had a mastectomy or who have very small breasts may find silicone gel implants to be a better choice.
Your Decision
Don’t feel dismayed by the wide range of implant choices available. The physicians at Marina Plastic Surgery Associates have the experience to help you decide which one is right for you. You can receive a free consultation when you request your appointment online and schedule during one of the convenient times they have set aside exclusively for Web visitors.